Oxidation Number of Chlorine
Write oxidation numbers if each atom close to the atoms. Difference between Valency and Oxidation Number.

How To Find The Oxidation Number For Cl In Cl2 Chlorine Gas Youtube
Oxidation and Reduction are two different things.

. The oxidation number is the hypothetical charge of an atom in a molecule or ion and it is a measure of its apparent capacity to gain or lose electrons within that species. Thus the valency of nitrogen is 3 whereas it can have oxidation numbers from -3 to 5. In fact they are opposites.
Man-made chlorine is commercially manufactured through the electrolysis of sodium chloride solution. When we look at the molecular weight chlorine dioxide contains 263 available chlorine. Oxidation Definition Oxidation is the loss of electrons by an atom molecule or ion Often the lost electrons are replaced by oxygen.
With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. Chlorine bromine and iodine usually have an oxidation number of 1 unless theyre in combination with oxygen or. The sum of oxidation numbers in a neutral compound is 0.
So lets quickly discuss what oxidation and reduction mean. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H. The chlorine atom in chlorine dioxide has an oxidation number of 4.
Chlorine is reduced to -1 oxidation state from 0 oxidation state. Check the number of atoms of oxidized and reduced in the reaction left and right side and balance them if they are not balance as the next step. At higher pH levels in the water chlorine becomes less effective and loses much of its oxidation potential requiring more chlorine or non-chlorine oxidizers to reach the same ORP levels.
Reduction is the gain of electrons or a decrease in the oxidation state of a chemical or atoms within it. Naturally occurring it is found in the mineral form of sodium chloride common salt and other salts. For instance the OS of iron in Fe_3O_4 is valued at 83.
107 Oxidation Reactions of Alkenes. Oxidation is the loss of electrons or an increase in the oxidation state of a chemical or atoms within it. Advanced Oxidation Processes AOPs are treatment technologies aimed at degrading and mineralizing recalcitrant organic matter from wastewater through reaction with hydroxyl radical OH.
Answers to Chapter 10 Practice Questions. Redox reductionoxidation ˈ r ɛ d ɒ k s RED-oks ˈ r iː d ɒ k s REE-doks is a type of chemical reaction in which the oxidation states of substrate change. Chlorine is a chemical element with atomic number of 17 and molecular mass of 355.
For this reason chlorine dioxide accepts 5 electrons when it is reduced to chloride. 107 Oxidation Reactions of Alkenes Alkenes undergo a number. Free chlorine when used for disinfection forms when chlorine gas is dissolved.
It also means that the compound will be readily available. Valency is different from the oxidation number and it has NO SIGN. When iodine chlorine and bromine are combined with oxygen their oxidation.
Addition of Bromine and Chlorine to Alkenes. 106 Two Other Hydration Reactions of Alkenes. In pool water chemistry once added to the water all chlorine oxidizers including salt produce hypochlorous acid HOCl hypochlorite ions OClᐨ and a byproduct specific to the type of.
Values are given for typical oxidation number and coordination. The donation of an electron is then 1. Keeping the atomic orbitals when assigning oxidation numbers in mind helps in recognizing that transition metals pose a special case but not an exception to this convenient method.
First ionisation energy The minimum energy required to remove. While fully ionic bonds are not found in nature many bonds exhibit strong. Electron affinity The energy released when an electron is added to the neutral atom and a negative ion is formed.
The sum of the oxidation numbers in a monatomic ion is equal to the overall charge of that ion. Free chlorine has a high oxidation potential and is a more effective disinfectant than other forms of chlorine such as chloramines. Oxidation potential is a measure of how readily a compound will react with another.
A high oxidation potential means many different compounds are able to react with the compound. Before moving on to more about oxidation number or state take a brief look at the process of oxidation. An atom that accepts an electron to achieve a more stable configuration is assigned an oxidation number of -1.
At times the OS can also be represented as a fraction. We get the following two definitions from this source. Recently these technologies have been proposed as a solution to treat emerging contaminants especially pharmaceuticals and personal care products Pignatello et al 2006.
The oxidation potentials of. The oxidation number of any atom in its elemental form is 0. Fluorine and other halogens have an oxidation number 1 when they appear as halide ions in their compounds.
Electronegativity Pauling scale The tendency of an atom to attract electrons towards itself expressed on a relative scale. One could thus define oxidation number or state by putting a value to such electron losses during a reaction which usually stood as integers. 105 Reaction of Alkenes.
This is more than 25 times the oxidation capacity of chlorine. To balance them make two. In chemistry the oxidation state or oxidation number is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionicIt describes the degree of oxidation loss of electrons of an atom in a chemical compoundConceptually the oxidation state may be positive negative or zero.
Atomic number17 atomic weight35446 to 35457 melting point103 C 153 F boiling point34 C 29 F density 1 atm 0 C or 32 F. Its monatomic form H is the most abundant chemical substance in the Universe constituting. The oxidation number of fluorine is always 1.
Chlorine is a toxic corrosive greenish yellow gas that is irritating to the eyes and to the respiratory system. In left side there are two chlorine atoms. Chlorine Cl chemical element the second lightest member of the halogen elements or Group 17 Group VIIa of the periodic table.
104 Reactions of Alkenes. Hydrogens oxidation number is 1 excluding when it is bonded to metals containing two elements. For example CaH 2 its oxidation number equals to 1.
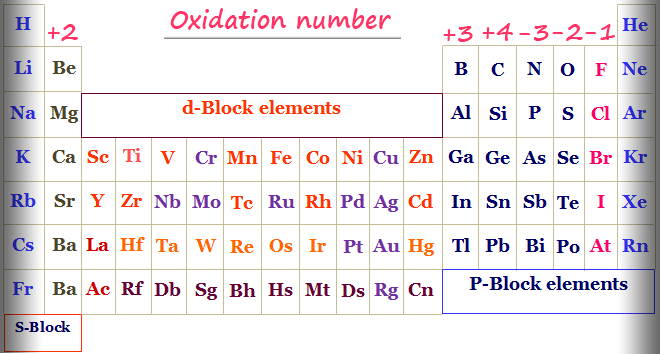
Oxidation Number Periodic Table Elements Definition Rules
Oxidation State Of Oxygen Top Sellers 51 Off Www Ingeniovirtual Com

Question Video Deducing The Oxidation State Of Chlorine In The Hypochlorite Ion Nagwa

How To Find The Oxidation Number For Cl In Cl2 Chlorine Gas Youtube
Comments
Post a Comment